Travere Therapeutics Inc Share News
Travere Therapeutics Inc Share News
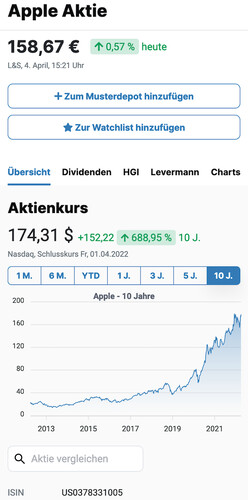
$8.28
0.73%
yesterday
Nasdaq,
Jul 01, 10:00 pm CET
Travere Therapeutics Inc Share News
Neutral
PRNewsWire
●
2 months ago
First non-immunosuppressive therapy for the treatment of IgA Nephropathy (IgAN) approved in Europe Conditional marketing authorization is based on statistically significant and clinically meaningful results from the phase-III PROTECT trial ST. GALLEN, Switzerland and SAN DIEGO, April 24, 2024 /PRNewswire/ -- CSL Vifor and Travere Therapeutics, Inc., (NASDAQ: TVTX) today announced that the Europ...
Neutral
GlobeNewsWire
●
3 months ago
Travere to present abstracts at SIMD and GMDI highlighting research investigating pegtibatinase as the first potential disease-modifying therapy for HCU
Neutral
GlobeNewsWire
●
3 months ago
SAN DIEGO, April 03, 2024 (GLOBE NEWSWIRE) -- Travere Therapeutics, Inc., (Nasdaq: TVTX) today announced that the Company will present nine abstracts in rare kidney disease at the World Congress of Nephrology (WCN) in Buenos Aires, Argentina, on April 13-16, 2024, and the American Nephrology Nurses Association (ANNA) National Symposium in Orlando, Florida, on April 14-17, 2024. At WCN, the Comp...
Neutral
GlobeNewsWire
●
4 months ago
SAN DIEGO, March 13, 2024 (GLOBE NEWSWIRE) -- Travere Therapeutics, Inc. (NASDAQ: TVTX) today announced that on March 10, 2024, the Compensation Committee of its Board of Directors granted inducement equity grants covering an aggregate of 51,600 shares of its common stock to four new employees, consisting of inducement stock options to purchase an aggregate of 25,000 shares of common stock and ...
Neutral
GlobeNewsWire
●
4 months ago
Travere announces submission of an sNDA to the FDA for conversion of existing US accelerated approval of FILSPARI (sparsentan) in IgAN to full approval.
Neutral
GlobeNewsWire
●
4 months ago
SAN DIEGO, Feb. 26, 2024 (GLOBE NEWSWIRE) -- Travere Therapeutics, Inc. (NASDAQ: TVTX) today announced that Company management will participate in the following upcoming investor conferences in March:
Neutral
GlobeNewsWire
●
4 months ago
Committee for Medicinal Products for Human Use (CHMP) recommends approval of the conditional marketing authorization (CMA) for sparsentan for the treatment of IgA nephropathy (IgAN) in Europe
Neutral
PRNewsWire
●
4 months ago
Committee for Medicinal Products for Human Use (CHMP) recommends approval of the conditional marketing authorization (CMA) for sparsentan for the treatment of IgA nephropathy (IgAN) in Europe Positive CHMP opinion is based on pivotal phase-III PROTECT study results European Commission decision is expected in Q2 2024 ST. GALLEN, Switzerland , Feb. 23, 2024 /PRNewswire/ -- CSL Vifor and Travere T...
Register for free
StocksGuide is the tool for easily finding, analyzing and monitoring shares. Learn from successful investors and make well-founded investment decisions. We make you a self-determined investor.